Arthrospira for Enhancing Resilience Against Viral Infections
Arthrospira as a Dietary Supplement and Food
Arthrospira, common name “spirulina”, is a cyanobacterium that has been used as a food for centuries and more recently as a health supplement by a large segment of global society. Since the early 1970’s, specific taxa have been cultivated on an industrial scale under control conditions. The commercially grown taxa have a distinct cell morphology, ultrastructure, ecology and gene sequence, and hence it has been proposed that a new genus, Limnospira, be established to include these popular species (Nowicka-Krawczyk et al, 2019).
Cultivation facilities exist throughout the world that provide a sustainable source of product material, for example Earthrise Farms in the US has an annual production of about 550 tons (Shao et al, 2019). Although early interest in commercial production of Arthrospira was focused mainly on its nutrient and protein content, it has emerged as a popular dietary supplement due to substantial scientific evidence supporting various human health benefits such as immune-enhancing and antiviral properties. Historical records indicate that Arthrospira was consumed by the Aztecs in Mexico and tribes around Lake Chad in Africa, where it is still used as a food today in the range of 10 to 40 g person/day (Abdulqader et al, 2000; Bigagli et al, 2017). Dietary supplement manufacturers recommend a daily dose of 3-6 g and some herbalists recommend doses of 10–20 g/day for athletes or individuals experiencing stress or an illness (Pitchford, 1996). Supporting its safe traditional use, no toxic effects have been observed in chronic or sub-chronic toxicity studies (Sánchez et al, 2003). For example, in a recent study using rats, Arthrospira was found to be safe and well-tolerated even when fed at a high dose (12 g/kg body wt/day) for 1 month (Bigagli et al, 2017). Arthrospira has also received GRAS status (GRN 000127) from the FDA.
Identification of Immune-Enhancing Compounds in Arthrospira and Development of Immulina™
In the late 1990’s, the National Center for Natural Products Research (NCNPR) established a program to investigate the immune-enhancing properties of botanicals. An initial assay was developed for the sensitive detection of extracts that enhanced innate immune cell function (activation of NF-kappa B in THP-1 human monocytes). Out of these efforts extracts from Arthrospira were found to be hundreds of times more active than all other samples tested.
Initial research at the NCNPR to identify the compounds responsible for the immune-enhancing activity of Arthrospira was driven by bioactivity-guided fractionation. The in vitro monocyte/macrophage stimulatory activity was found to reside in a high molecular weight fraction that was predominantly composed of complex polysaccharides (Pugh et al, 2001). Building on this discovery an aqueous ethanol extract, called “Immulina™”, was developed to enrich the level of immune-enhancing compounds from Arthrospira (United States Patents 7,205,284 and 7,846,452).
During efforts to optimize extraction conditions it was observed that preparations exhibiting dramatically different levels of in vitro activity had no significant differences in the structural composition of the high molecular weight polysaccharides. Furthermore, a detailed literature analysis indicated that a variety of different polysaccharides have been isolated from Arthrospira that are purported to be responsible for macrophage activation. This suggested that either a diverse array of polysaccharides having complex structure-activity relationships was responsible for the immune-enhancing properties of Arthrospira, or another class of macromolecules, that co-purifies with the polysaccharide fractions, was responsible for the observed activity.
To investigate whether a non-polysaccharide macromolecule could be responsible for the activity detected in Immulina™, a variety of biochemical approaches (enzyme digestions, inhibitors, and chemical treatments) were employed. The activity of Immulina™ was unaltered by treatment of various proteases, nucleases, or elevated temperature (100°C for several hours). However, exposure to hydrogen peroxide, NaOH and lipoprotein (diacyl glycerol) lipase destroyed all monocyte-stimulating activity, indicating that a macromolecule containing a lipid moiety was essential for activity. Furthermore, NCNPR research demonstrated that the immune-enhancing compounds in Immulina™ activated innate immune cells through a Toll-like (TLR)2- and CD14-dependent process (Balachandran et al, 2006). Since Arthrospira is a prokaryotic cyanobacteria and bacteria are known to contain Braun-type lipoproteins that activate immune cells through a CD14- and TLR2-dependent process (Hashimoto et al, 2006), additional studies were conducted to determine if this class of compounds was responsible for the observed activity (Nielsen et al, 2010).
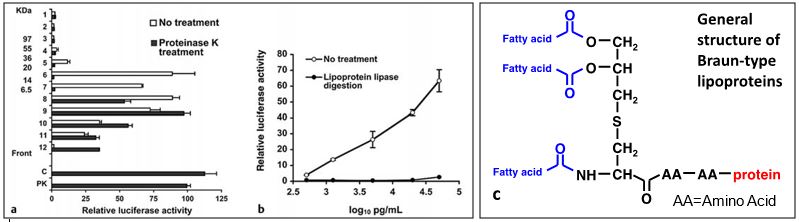
Figure 1a provides evidence that protein comprises the major portion of the active molecules. This is indicated by the decrease in overall size of the active molecules that is observed when the Immulina™ sample is digested with a protein degrading enzyme (proteinase K). Fractionation was performed on SDS polyacrylamide gel. The activity of the untreated (C) and proteinase K treated (PK) Immulina™ before gel electrophoresis were similar indicating that the majority of the protein component was not required for activity, similar to other characterized bacterial lipoproteins (Hashimoto et al, 2006; Aliprantis et al, 2001). The results presented in Figure 1b show that the activity present in Immulina™ is completely abrogated by removal of glycerol bound fatty acids by treatment with lipoprotein lipase. These results were replicated with other aqueous extracts of Arthrospira and provide robust evidence that bacterial Braun-type lipoproteins fully account for the in vitro macrophage stimulatory activation exhibited by Arthrospira. These lipoproteins contain a diacylglycerol moiety that is linked by a thioether bond to the N-terminal cysteine of the peptide chain (Figure 1c). Additional evidence that the active lipoproteins detected in Immulina™ are of the Braun type is indicated by the detection of the unique structural component 2,3-(dihydroxypropyl) cysteine within this extract (Nielsen et al, 2010).
The Immulina™ extract has been commercially available in the United States and Europe for the last 15 years and is sold as a botanical ingredient by ChromaDex. Stability studies conducted on Immulina™ indicate that there is no loss in in vitro monocyte-stimulatory activity over a ten-year period for product material stored at room temperature as a dry powder.
The Potency of Immulina™ is Standardized Based on Biological Activity
Botanicals can exhibit substantial variation in the level of their chemical constituents and this has contributed to a lack of consistent product efficacy for consumers and use in clinical trials. For example, our research during the past 15 years has demonstrated that the macrophage stimulatory activity of ImmulinaTM extracts prepared from different batches of Arthrospira raw material varies up to 20-fold. To address this quality control problem in botanicals, chemical standardization methods are being used to verify that the levels of active compounds (when known) within these products are consistent. However, the most commonly identified substances thought to be responsible for the health benefits of immune-enhancing botanicals are typically complex mixtures from families of high molecular weight components – such as the Braun-type lipoproteins in Arthrospira. Since the physiochemical properties of such macromolecular components do not cleanly predict or correlate with their biological activity, the accurate assessment of the potency of these compounds cannot be achieved by using only analytical techniques. An analogous problem existed in the field of pharmaceutical biologics, a problem eventually solved by bioassay-based standardization to afford consistent quality of products. Proper characterization of biologics therefore requires information from both physicochemical and bioassay methods. About 15 years ago, researchers at the NCNPR recognized the critical need to adapt the concept of biological standardization to botanicals to ensure accurate quantitation of the activity/potency of the high molecular weight bioactives present within these products. The immune-enhancing activity/potency of ImmulinaTM is standardized using an in vitro bioassay by testing the extract directly without the need for purification of the active Braun-type lipoproteins (which is a challenging and difficult process). In addition to using this bioassay to ensure product quality (batch-to-batch consistency), this system is also used to select batches of raw material, monitor production protocols, evaluate finished product formulations, and perform stability studies.
Site of action for Immulina™ – The Mucosal Immune System
Most pathogens penetrate the host system through mucosal surfaces. A complex network of cells and cellular structures, known as the mucosal immune system, has evolved within higher organisms as a front-line defense to protect these exposed surfaces from invasion by pathogens. The recognition of pathogens by innate and other immune cells located throughout the gut is mediated by a family of pattern-recognition receptors that include toll-like receptors (TLR) that recognize specific pathogen-associated molecular patterns. Ligands for these receptors include LPS (TLR4 agonist), bacterial Braun-type lipoproteins and lipoteichoic acid (TLR2 agonists), bacterial DNA (TLR9 agonist), bacterial flagellin (TLR5 agonist), and other pathogen components such as viruses and fungi. A major inductive site within the gut mucosal immune system are modified lymph nodes known as Peyer’s patches. Immunogenic material is transported into these structures from the intestinal lumen by M cells. Antigen presenting cells such as macrophages and dendritic cells within Peyer’s patches are thus exposed to intestinal material for potential recognition of pathogens or pathogen components such as Braun-type lipoproteins. Activation of dendritic cells and macrophages by stimulation of TLRs results in the production of cytokines that can influence the immune response towards resilience against a pathogen.
Research demonstrates that the composition of the intestinal microbiota is involved in regulating immune defense mechanisms against influenza A virus infection. For example, dramatic alteration in the species composition of intestinal bacteria within mice, resulting from oral antibiotic treatment, is associated with an impaired immune response against respiratory tract viral infection. Intestinal microbiota-mediated regulation of immune defense against viral infection appears to be due, in part, to commensal bacteria-derived components that activate TLRs within the host (Ichinohe et al, 2011). Additional evidence demonstrating that the presence of bacteria within the gut can impact the severity of influenza viral infection is provided by the scientific literature on orally administered probiotics. A growing body of literature, based on mouse studies, indicates that oral administration of probiotic bacteria prior to influenza viral infection results in a protective effect against viral-induced damage. Evidence suggests that the anti-viral effect is due to probiotic-derived bacterial components since protection against infection is observed after oral administration of live (Kawase et al, 2010) as well as heat-killed probiotics (Kawase et al, 2012). Furthermore, research indicates that the protective effect of orally administered probiotics against viral infection is mediated by activating the host immune system. For example, protective effect against influenza viral infection was only observed after oral administration of a Lactobacillus plantarum strain inducing high cytokine production in vitro whereas no protection was observed for strains inducing low cytokine production or inhibition of cytokine secretion (Kechaou et al, 2013). In agreement with this probiotic literature, we have previously demonstrated that oral administration of Immulina™ is able to exert a protective effect against influenza virus infection in mice (Pugh et al, 2015).
Since the proximal small intestines has orders of magnitude less microbes as compared to the colon, this is likely the main site of action for orally administered bacterial components that are present in products such as probiotics and Immulina™. It is unlikely that oral administration of TLR ligands would induce a significant effect in the colon since this section of the gut already contains a high concentration of bacterial components. Our previous research demonstrating that oral administration of Immulina™ to mice increases ex vivo production of IgA and IL-6 in Peyer’s patches of the small intestines (Balachandran et al, 2006) provides supporting evidence that the small intestines is a major site of action for the immune-enhancing effects of Arthrospira products.
Summary of In Vitro Data on Immune-Enhancing Activity of Immulina™
The NCNPR has evaluated the in vitro immune-enhancing activity exhibited by numerous batches of Immulina™ extracted from Arthrospira raw material obtained from cultivation facilities throughout the world. Although activity can vary between different batches, extracts consistently exhibits potent activation of monocytes/macrophages.
Table 1: Summary of In Vitro Data for Immulina™
| Activation of Innate Immune Cells | |||
| Study 1: THP-1 Monocytes | Study 2: THP-1 Monocytes | Study 3: RAW 264.7 Macrophages | |
|
|
|
|
| Mechanism and Identification of Braun-Type Lipoproteins | |||
| Study 4: THP-1 Monocytes | Study 5: THP-1 Monocytes | ||
|
|
||
Immulina™ activates the NF-kappa B signaling pathway and multiple in vitro studies have demonstrated enhanced mRNA and protein levels of various cytokines in monocyte and macrophages (Study 1, Study 2, Study 3). Using various biochemical approaches, it was discovered that the monocyte/macrophage stimulatory of Immulina™ was due to bacterial Braun-type lipoproteins (Study 4). Bacterial lipoproteins of the murein type are produced by both gram-negative and gram-positive bacteria and are thought to be unique to prokaryotes (Arthrospira is a prokaryotic cyanobacteria). Bacterial Braun-type lipoproteins are known to activate immune cells through a TLR2-dependent pathway. Similarly, we have reported that activation of innate immune cells by the Arthrospira Braun-type lipoproteins are dependent on TLR2 but not TLR4 signaling. This was demonstrated using two different approaches – blocking antibody studies and expression vector experiments (Study 5). Results of these studies are also noted in Table 1.
Summary of In Vivo Data on Enhancing Resilience Against Viral Infections by Arthrospira Products
Using a well-established animal model, NCNPR investigators have demonstrated that oral administration of Immulina™ by mice (30 day pre- and 21 days post-infection) substantially reduced several pathological parameters due to influenza A (H1N1) infection. The most dramatic differences observed between the treatment and control mice were with respect to weight loss, an indicator of morbidity, and lung histopathology, an assessment of damage due to viral replication as well as lymphocyte infiltration (Study 6). The results of this mouse model indicate that the Immulina™ extract enhances host resilience to successfully withstand and mitigate the effect of influenza viral infection. Adding support to the Immulina™ study, other investigators have demonstrated that mice fed a cold-water extract of Arthrospira starting 4 hours pre- and continuing 4 days post-influenza A (H1N1) infection showed a dose-dependent improvement in survival rate (Study 7), indicating effective recovery/resistance to viral challenge. A cold-water extract of Arthrospira will contain the active Braun-type lipoproteins, although optimal levels are achieved with an aqueous alcohol extract (conditions used for preparation of Immulina™). Consistent with these mouse studies, research using chickens reported that, although no difference was observed with myocardial necrosis, there was decreased mortality caused by influenza H5N1 virus in animals that orally consumed Arthrospira at 20% in the drinking water for 19 days prior to infection (Study 8). Results of these studies are also noted in Table 2.
Table 2: Summary of Animal Model Data on Arthrospira Products
| Resilience Against Influenza Viral Infection | ||
| Study 6: Immulina™ Extract | Study 7: Cold Water Extract | Study 8: Arthrospira |
|
|
|
| Changes in Immune Parameters | ||
| Study 9: Immulina™ Extract | ||
| ||
The effect of oral administration of Arthrospira extracts against influenza viral infection is probably mediated by activation of various innate and adaptive host immune defense mechanisms. In a separate study involving healthy mice, oral administration of ImmulinaTM enhanced the ex vivo production of IgA and IL-6 from Peyer’s patch cells as well as interferon-gamma production from spleen cells (Study 9). Enhanced IgA production may be involved in mediating the protective effects ImmulinaTM against influenza viral infection since this antibody prevents the adherence of viruses to mucosal surfaces and is important in eliminating infectious agents.
Summary of Human Trials on Arthrospira Products Demonstrating Reduction of Various Viral Infections and Increased Natural Killer Cell Activity
Based on anecdotal reports that consumption of ImmulinaTM prevents cold sores, a small randomized, double blind, placebo-controlled study was conducted (Study 10, unpublished). The results indicated that consumption of Immulinaä (400mg/day) for 4 months significantly reduced the number of recurring outbreaks of herpes simplex virus. In a separate randomized, multi-center study, consumption of Arthrospira raw material for 6 months (10g/day) resulted in a decreased viral load in HIV ART-naïve subjects (Study 11). These two studies (see Table 3) provide hypothesis-generating data that consumption of Arthrospira products may have beneficial effects for viral infections in general and lends support for the potential that Immulina™ could enhance resilience against respiratory viral infections.
To investigate the effect of oral administration of ImmulinaTM on possible immune targets in human subjects, two small-scale pilot studies were conducted. Dietary supplementation with Immulinaä (400mg/day) for one week by healthy volunteers enhanced NK cell activity an average of 40% and 54%, respectively, for the two studies (Study 12). Similar results on enhancement of NK activity have also been reported by other investigators using a very small number of subjects that orally consumed 50mls of a hot water extract of Arthrospira for various lengths of time (Study 13). A hot-water extract of Arthrospira will contain the active Braun-type lipoproteins, although optimal levels are achieved with an aqueous alcohol extract (conditions used for preparation of Immulina™). In another clinical study conducted in health elderly subjects, no significant effects were observed on T cell numbers (Study 14). Results of these studies are also noted in Table 3.
Table 3: Summary of Human Trials with Arthrospira Products
| Anti-Viral Effects | |||
| Study 10: Immulina™ (unpublished) | Study 11: Arthrospira Powder | ||
|
| ||
| Changes in Immune Parameters | |||
| Study 12: Immulina™ | Study 13: Hot Water Extract | Study 14: Immulina™ (ClinicalTrials.gov Identifier: NCT01784692 | |
Trial 1: Pilot Experiment
|
|
|
|
Taken together, these human subject trials along with the mouse studies described in Table 2, indicate that enhancement of NK cell activity plays an important role in the immunomodulatory action of ImmulinaTM and is likely involved antiviral host resilience since there is increasing evidence that NK cells serve a critical role in influenza infection by killing infected cells to contain viral replication (Schultz-Cherry 2015).